ILSOYADVISOR POST
Part 2 – Soybean Calcium Requirements: Understanding the Calcium Cycle
Soil tests indicate large amounts of exchangeable calcium in the soil, but what’s important is how much soluble calcium is present. You can ask to have a water extraction done to measure soluble calcium in the soil solution. If you have both chemical and water extracts done, you will notice that soluble calcium is just a fraction of exchangeable calcium—that is because of the calcium and its interaction in the soil.
Crop nutrients follow a cycle that tracks how they enter the soil and their fate once there. There are a carbon, nitrogen, phosphorus, potassium and sulfur cycles that are well understand. However, other minerals like calcium and magnesium also follow a cycle, but it’s less recognized.
Understanding these cycles helps you manage nutrient mineral and understand how it interacts with the soil. Figure 1 explores the cycle of calcium in the soil and how it interacts with its environment. And calcium, like all nutrient is unique, click here to read more about calcium in the soil.
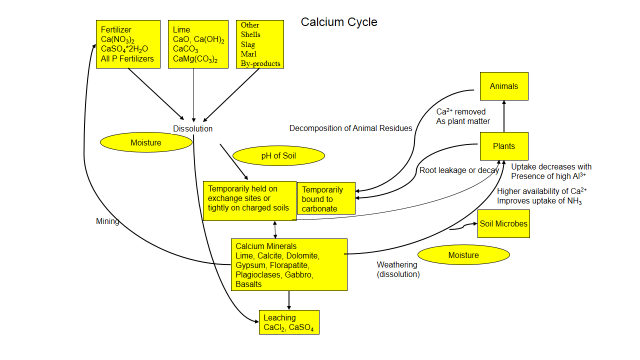
Figure 1. Calcium Cycle
Importance of calcium
Plants take up calcium as a divalent cation Ca+2. In plants it moves in the xylem tissue with water, up through the plant through stems and petioles to leaves. It is considered immobile in the plant and once it reaches its destination and use, it can’t be remobilized and moved to meet new growth demands. Calcium is required for cell wall rigidity, cell division of meristems and root tips, membrane function and integrity, acts as a secondary messenger or signaling compound, actively involved in sugar transport, etc.
Forms of calcium in the soil
Calcium is present in the soil as calcite and phosphate minerals or other minerals in the soil and isn’t readily available. Through natural physical and chemical weathering, calcium is slowly released by dissolution and becomes soluble. Soluble calcium attaches to soil’s exchange sites or binds with phosphate and carbonate and becomes insoluble. Calcium can also be added as fertilizer, lime, gypsum or other by-products. And it’s only slightly mobile in the soil, generally adhering to soil particles, carbonates or phosphates, preventing movement.
Large amounts of calcium are held on soil’s exchange complex while smaller amounts are in the soil solution. Both forms are available to plants and soil organisms. When absorbed by plants or microorganisms, calcium enters an organic phase. The calcium is a constituent of walls and plays a role in metabolic processes. When a plant, animal or soil fauna dies, decomposers break down the organism and calcium is released back to the soil. Calcium is continually recycled between plant roots, microorganisms and soil. But its fate is influenced by the chemistry around it.
Impact of soil chemistry on calcium
Calcium is influenced by soil pH and is more available from a pH of 7 .0 to 8.5. Soils with a pH above 7.5 can contain free lime as calcite flakes that are often very visible. Calcium competes with other positively charged ions, such as sodium (Na+1), potassium (K+1), and magnesium (Mg+2). Applying too much of these positively charged ions can decrease calcium uptake by plants. Sodium ions can replace the adsorbed calcium, damage soil structure and decreases calcium availability.
The University of Missouri’s Dr. William Albrecht evaluated base saturation and experimented with different cation ratios and concluded that the idea saturation was 65-68 percent Ca, 12-15 percent Mg, 4-5 percent K, and 1-5 percent Na with the rest being hydrogen (H). And calcium will easily precipitate as a mineral. Soluble calcium in the soil forms insoluble compounds with phosphorous and carbonates. Consequently, calcium and phosphorous availability are both diminished.
While it appears in soil tests that the soil has plenty of calcium, maintaining more of that calcium in a soluble phase is a worthwhile goals.
Soybean agronomist Daniel Davidson, Ph.D. posts blogs on agronomy-related topics. Feel free to contact him at djdavidson@agwrite.com or ring him at 402-649-5919.





Comments
Add new comment