ILSOYADVISOR POST
SRIN: New Biotechnology Rules Will Bring Better Soybeans, Faster
The U.S. Department of Agriculture (USDA) has completely overhauled the process used to regulate new genetic material, noting it will “reduce regulatory burden for developers of organisms that are unlikely to pose risks.” It also allows regulators to focus resources and oversight on areas with the greatest potential for risk. This win-win will continue to protect plant health while increasing regulatory precision.
“We are always looking for new and better seeds, to grow new and better soybeans. Any process that gets improved seeds into our hands and our fields more quickly is helpful,” says Tom Oswald, United Soybean Board Director and Iowa soybean farmer.
Beginning in May 2020, the USDA’s Animal and Plant Health Inspection Service (APHIS) began phasing in new regulatory procedures for plants developed using biotechnology. Specifically, these rules govern the transportation and controlled release of such organisms. This is the first comprehensive revision of APHIS’ biotechnology rules since 1987, when they were initially established. The completely new approach takes advantage of over 30 years of experience and scientific advances to build a clear, consistent, science- and risk-based regulatory framework. By allowing now-established science to guide the process, APHIS incorporates advances in both the science of genetic engineering, and in understanding of the plant pest risks posed by organisms developed using this technology.
APHIS’ mission continues to be to protect agricultural and natural resources, and to ensure that newly developed organisms do not pose a pest risk to plants. A pest risk is defined as something that can directly or indirectly injure, cause damage to, or cause disease in any plant or plant product. The new process allows regulators to focus their resources on areas where risks are most likely, and reduce regulatory burdens from products with low risk.
The new rule, called Sustainable, Ecological, Consistent, Uniform, Responsible, Efficient (SECURE), replaces the previous “Am I Regulated?” and Extension processes and regulates the importation, interstate movement, and environmental release of plants and other organisms. However, the new rules focus on the properties of the organism itself, and not on the method used to produce the organism.
The new rules still require a permit for any plant or organism subject to regulation that is moved across state lines or that is released into the environment (including confined field trials). Applications for movement or environmental release permits will be approved quickly, and APHIS will continue to conduct inspections on compliance and records.
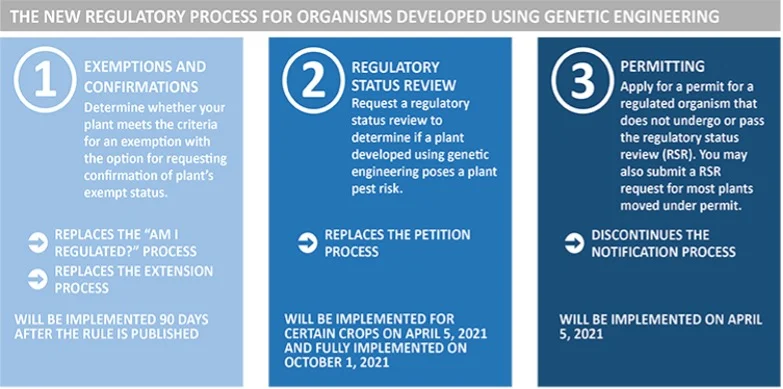
Under the new procedures, there are three types of plants that are exempt from regulation, mainly plants that were modified in ways that could have been achieved through conventional breeding. The scientific rationale behind this is that genetic engineering, in and of itself, has not been shown to introduce plant pest risk, and regulators can use what is known about conventionally bred plants to inform decisions about genetically modified plants. The exceptions are specifically defined in the statute, or a developer can ask for a Regulatory Status Review (RSR) for a bioengineered plant they have developed, to determine whether or not a proposed plant is subject to the regulations.
When an RSR is requested, the proposed plant will be evaluated based on its biological properties, the new trait or characteristic, and the mechanism of action (or how the developer caused the new trait to occur). The proposed new plant will be evaluated relative to existing plants to determine if there is a plausible pathway to an increased plant pest risk. If a plausible pathway is identified, the developer may request an evaluation of the likelihood and consequences of this increased risk. That evaluation would include agency review, results publication and solicitation of comments from the public, and then a final determination by APHIS as to whether or not the newly developed plant is subject to the regulations. The new regulations are being phased in to allow time for developers and information systems to be changed to comply with the new rules. APHIS will begin accepting new RSR requests for soybeans and other crops in April 2021, and will be fully implemented for all crops by October 2021.
In the future, USDA may regulate not only plants but also animals. In December 2020, USDA started soliciting comments from the public on what would happen if regulation of animals developed using biotechnology was moved from the FDA to APHIS (https://www.aphis.usda.gov/brs/pdf/aphis-2020-0079.pdf).

For more details about the new rules, visit https://www.aphis.usda.gov/aphis/ourfocus/biotechnology/biotech-rule-revision/secure-rule/secure-about/340_2017_perdue_biotechreg.
This article was originally published on soybeanresearchinfo.com. To view the original article, click here.




Comments
Add new comment